Pipeline

Advancing a suite of precision psychiatric medicines
Our drug candidates are designed to target core brain processes in patient populations not adequately treated by currently available medications.
We aim to address specific phenotypes of broader neuropsychiatric diagnoses, with an initial focus on major depressive disorder (MDD), bipolar depression, and schizophrenia.
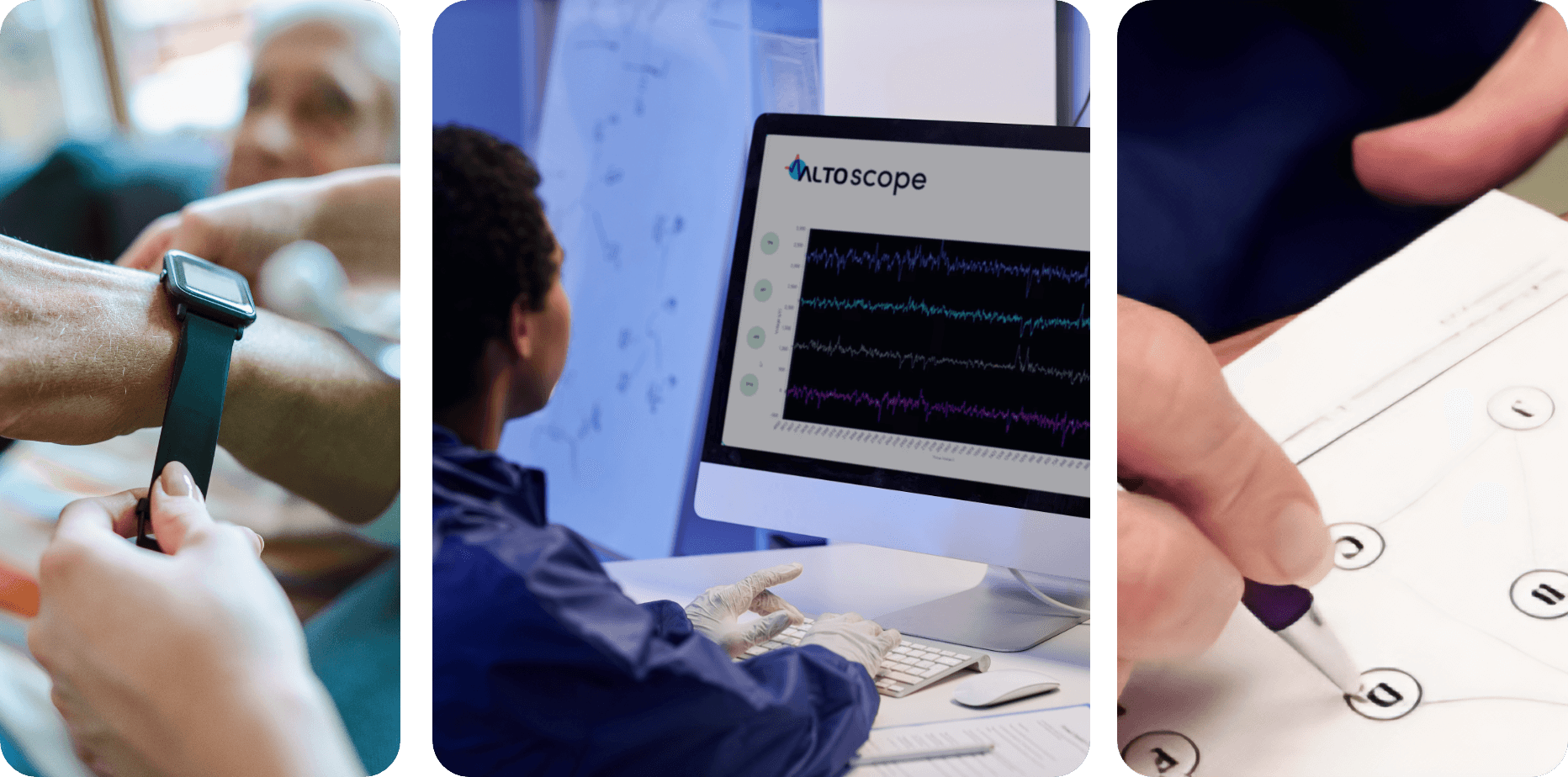
Aiming to improve clinical outcomes and reduce development risk
Our Precision Psychiatry Platform™ generates biological and clinical insights that are rarely, if ever, gained through traditional CNS drug development approaches. This information directly guides each phase of the drug development process. We aim to use these unique insights to increase the probability of clinical success for our drug programs and thereby drive meaningful impact for patients.
Our pipeline
mid-2026
1H 2025
2H 2025
Clinical programs
Our clinical-stage candidates build on positive results in targeted patient populations.
ALTO-100
ALTO-100 is a first-in-class, oral small molecule believed to work through enhancing neural plasticity in the hippocampus, a brain region important for both cognition and mood. It builds on a long-standing hypothesis that depression is in part driven by a reduction in hippocampal neuroplasticity and as such is targeted to those patients who show a cognitive profile indicative of this neuroplasticity deficit. ALTO-100 is currently in Phase 2b development for the treatment of bipolar depression as an adjunctive treatment.
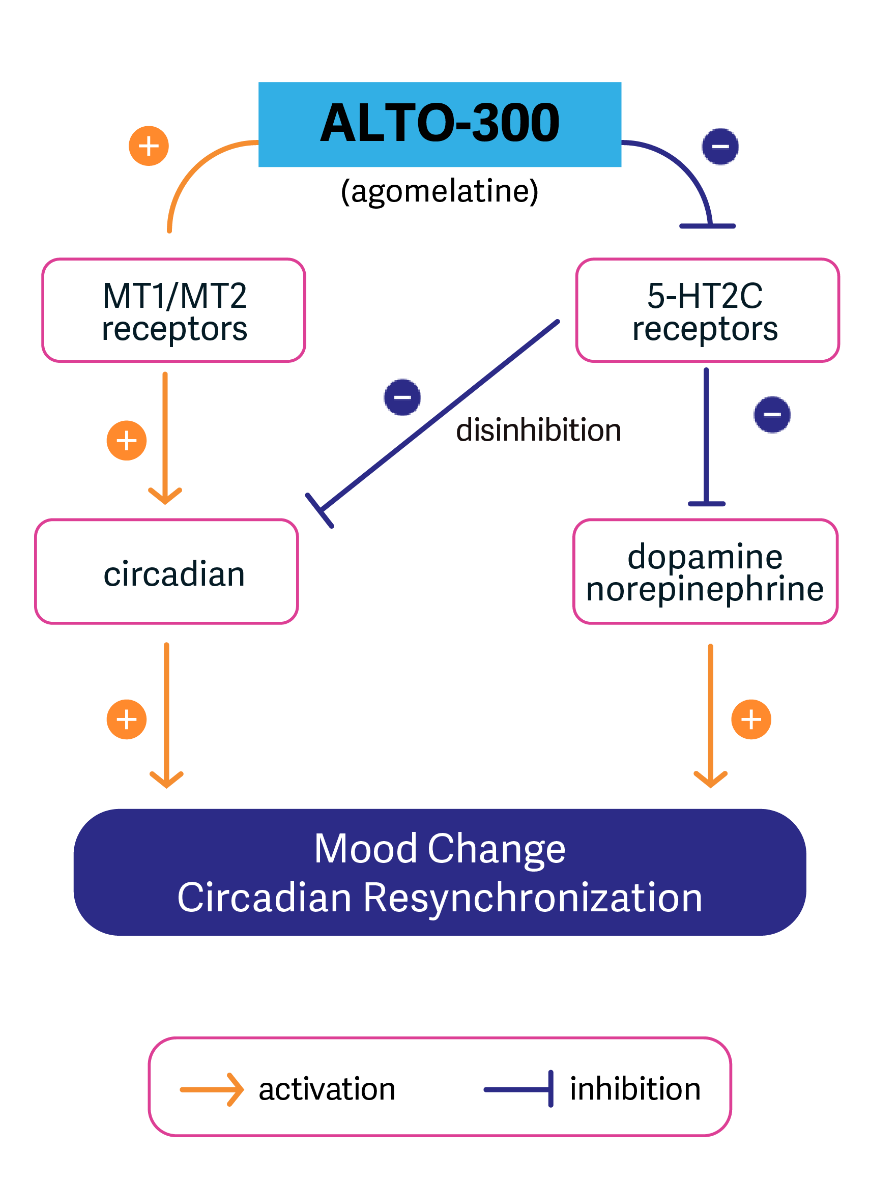
ALTO-300
ALTO-300 is a small molecule melatonergic agonist and serotonergic antagonist (which increases dopamine and norepinephrine levels) being developed as an adjunctive treatment in the United States for patients with MDD. ALTO-300 is a drug with validated antidepressant efficacy and was approved for the treatment of MDD in Europe and Australia, but has not been approved in the United States. We are developing it in an EEG biomarker-characterized patient population.
In a Phase 2a trial, ALTO-300 demonstrated significant promise as an adjunctive treatment for people with MDD who experienced inadequate response to an antidepressant. Treatment with ALTO-300 led to a significantly greater improvement in MADRS scores in a patient population with MDD characterized by a machine learning derived EEG biomarker.
Driven by these results, we launched a double-blind, placebo-controlled, randomized Phase 2b trial involving EEG biomarker-characterized patients with MDD.
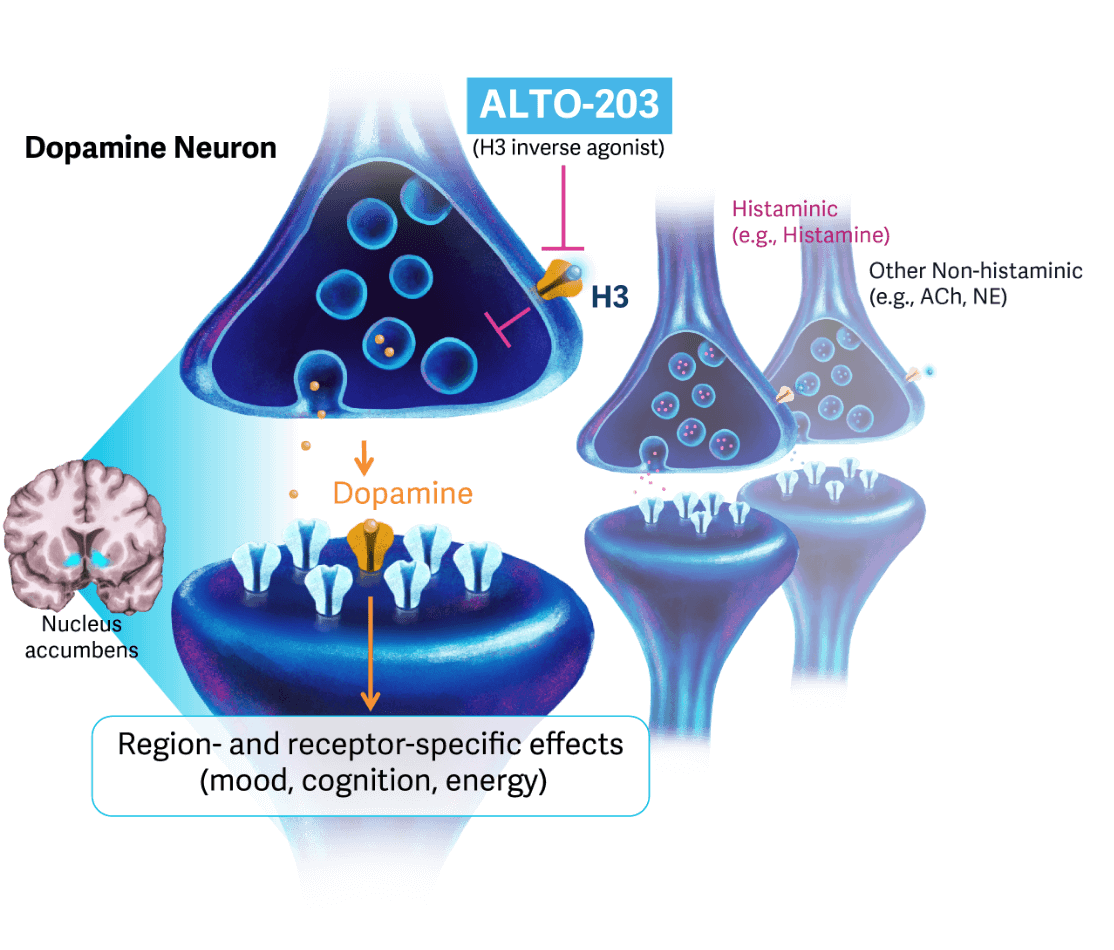
ALTO-203
ALTO-203 is a novel, oral small molecule histamine H3 receptor inverse agonist being developed for patients with MDD and higher levels of anhedonia as defined by a lack of motivation or pleasure. This compound was found to increase dopamine release in the reward system, an area of dysfunction important for anhedonia.
Building on favorable results from a Phase 1 trial in healthy individuals, we launched a Phase 2a double-blind, placebo-controlled, proof-of-concept study designed to determine ALTO-203’s benefits in patients with MDD and higher levels of anhedonia, focusing on the acute clinical impact of single doses of the drug.
ALTO-101
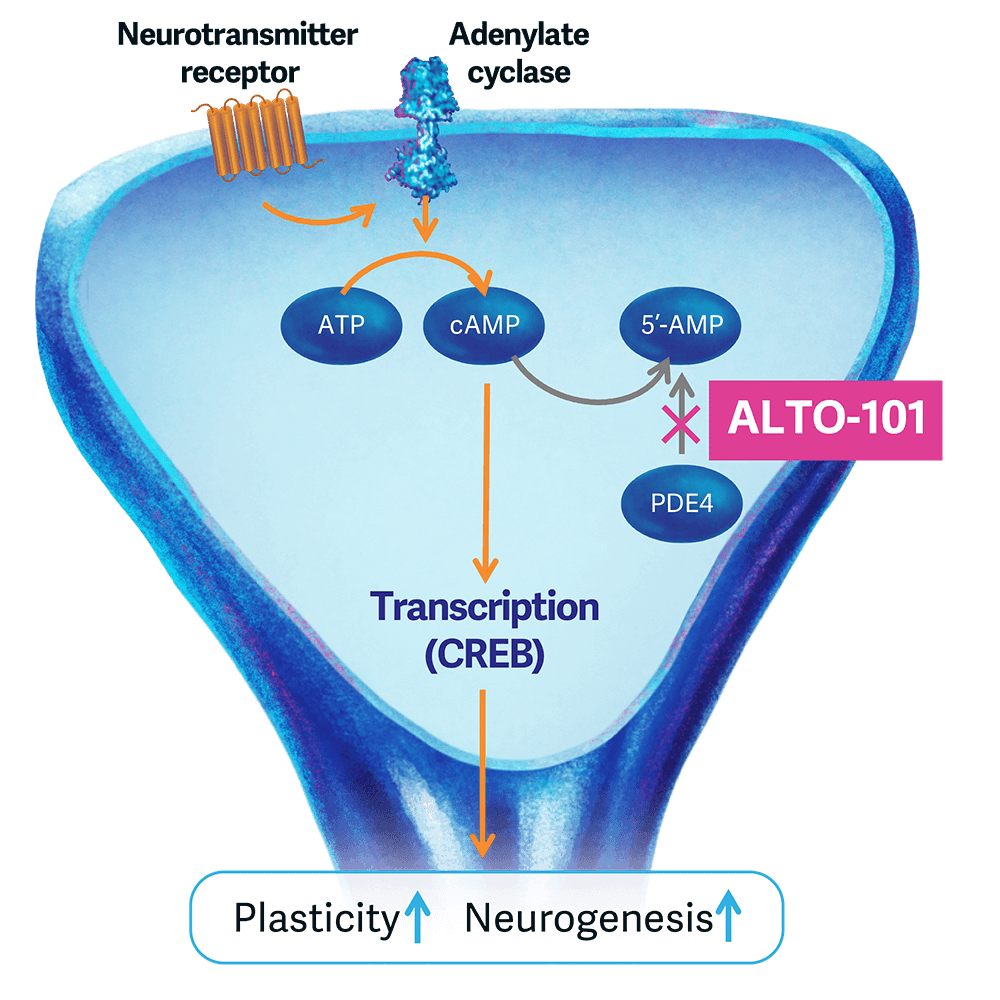
ALTO-101 is a brain-penetrant PDE4 inhibitor designed as a novel transdermal formulation being developed for patients with schizophrenia with cognitive impairment. PDE4 is an enzyme that breaks down cAMP, an intracellular signaling molecule important for cognition and neuroplasticity, which has long been of interest for its putative pro-cognitive and antidepressant effects.
Across two Phase 1 trials in healthy individuals, ALTO-101 demonstrated dose-response benefit on measures of cognitive processing, as well as favorable tolerability and improved pharmacokinetics when administered via a transdermal delivery system (TDS). The TDS formulation, as compared to traditional oral administration, resulted in substantially fewer class-related adverse events typically associated with PDE4 inhibitors, allowing us to administer dosage levels guided by our EEG and behavioral biomarker readouts rather than be limited by the kind of adverse events that have challenged use of PDE4 inhibitors across various indications. Our ongoing Phase 2 proof-of-concept study in adults with schizophrenia, which consists of a cross-over, double-blind, placebo-controlled, dose-escalating treatment with ALTO-101 will assess the impact on EEG measures of cognitive processing, as well as cognition itself.
ALTO-202
ALTO-202 is an orally bioavailable negative allosteric modulator of the GluN2B subunit of the NMDA receptor. The mechanism of ALTO-202 builds on the antidepressant effects observed with NMDA receptor antagonists like ketamine, but does so as an oral drug that has not exhibited the common dissociative adverse effects. ALTO-202 has demonstrated a favorable safety profile across various clinical populations.